4-Methoxyphenylboronic Acid CAS 5720-07-0 Purity >99.5% (HPLC) Factory High Quality
Manufacturer Supply With High Quality, Commercial Production
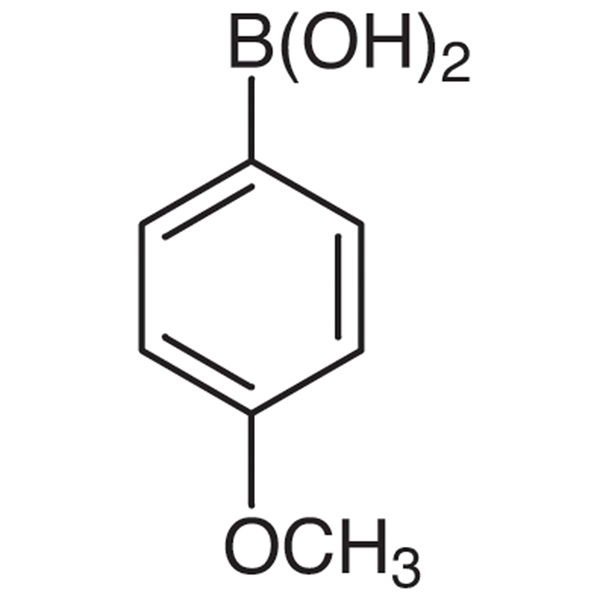
Chemical Name: 4-Methoxyphenylboronic Acid CAS: 5720-07-0
| Chemical Name | 4-Methoxyphenylboronic Acid |
| Synonyms | 4-Methoxybenzeneboronic Acid; p-Methoxyphenylboronic Acid; p-Methoxybenzeneboronic Acid; p-Anisylboronic Acid |
| CAS Number | 5720-07-0 |
| CAT Number | RF-PI1327 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C7H9BO3 |
| Molecular Weight | 151.96 |
| Melting Point | 204.0~206.0℃ (lit.) |
| Solubility | Soluble in Dimethyl Sulfoxide and Methanol; Slightly Soluble in Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Powder |
| Purity / Analysis Method | >99.5% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Single Impurity | <0.50% |
| Total Impurities | <0.50% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; OLED Intermediates |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


4-Methoxyphenylboronic Acid (CAS: 5720-07-0) is a reagent used for: Suzuki-Miyaura cross-coupling reactions; Pd-catalyzed direct arylation; Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water; Palladium-catalyzed stereoselective Heck-type reaction; Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence; Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides; Ruthenium catalyzed direct arylation; Rh-catalyzed asymmetric conjugate addition. 4-Methoxyphenylboronic Acid is used as organic synthesis intermediates, pharmaceutical intermediates and OLED intermediates, liquid crystal intermediates or display materials. 4-Methoxyphenylboronic Acid is a reagent used in the preparation of various biological inhibitors.